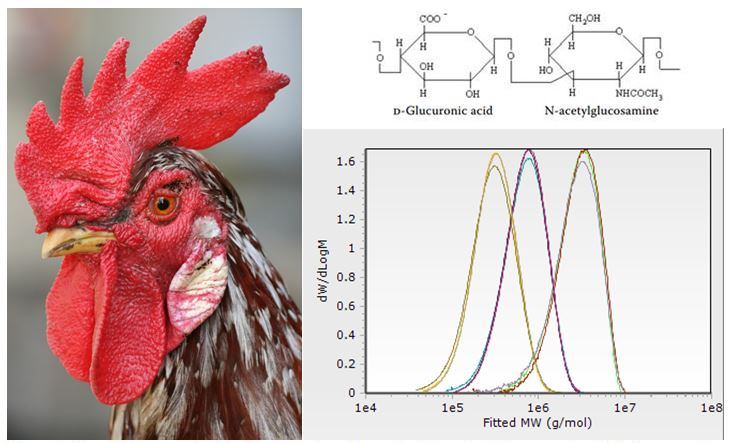
Figure 1: Top right—repeat unit of hyaluronic acid, a material particularly concentrated in (among other tissues) chicken combs (left). Bottom right—representative triplicate determinations of three samples of hyaluronic acid of distinct molecular weights.
Chemical Properties of Hyaluronic Acid
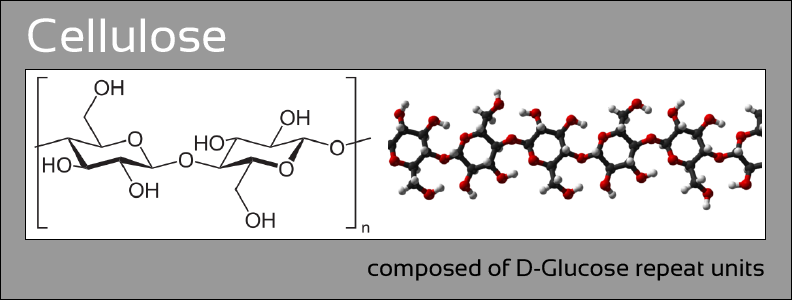
Hyaluronic acid (HA) is a naturally synthesized highly linear polysaccharide found in a variety of tissues including the joint space, vitreous humor, connective tissue, synovial fluid, and even chicken combs (Fig 1 left). Naturally synthesized HA is typically of a very high molecular weight well over 1,000,000 g/mol; it is extremely hydrophilic, highly lubricious, undergoes degradation in response to naturally occurring hyaluronidase enzymes, and binds to a variety of biological receptors. Such properties have driven interest in the use of HA in a variety of applications such as for the treatment of osteoarthritis knee pain, drug delivery/targeting, and tissue engineering.
In a physiological solution, the HA molecule is understood to exist in a twisted and entangled ribbon structure evolving from a combination of its repeat unit structure, internal hydrogen bonding effects, ionic interactions with the solvent, and its highly linear structure. These properties, in conjunction with the molecular weight of HA, have been shown to strongly affect its functional rheological properties, as has been reported by CPG scientists in the past.[1]
The accurate assessment of HA’s molecular weight therefore is an invaluable tool for applications such as material characterization, stability assessment, delivery behavior, and quality control. However, the same properties which lend to its unique properties also make molecular weight analysis of the material extremely challenging.
Molecular Weight Determination of HA
Polymer molecular weight is commonly determined by gel permeation chromatography (GPC; also known as size exclusion chromatography or SEC). However, due to the polymer’s extremely high intrinsic viscosity, large hydrodynamic volume, slow reptation rate, highly linear structure, and propensity for entanglement effects and shear degradation, analytical conditions must be chosen extremely carefully.
Some key analytical process variables which may dramatically impact measured molecular weights include sample concentration, dissolution time/temperature/agitation, solution handling, sample filtration (or lack thereof), mobile phase selection, injection volumes, flow rate, column selection, and calibration methodology. No conventional molecular weight calibration standards exist which may be used for HA analysis, and as a result, absolute molecular weight analysis by light scattering or triple detection is most commonly performed instead for accurate MW measurement.
CPG has validated an in-house method for the determination of hyaluronic acid molecular weight distributions by triple detection GPC (Fig 1, lower right). Contact us for additional information on this methodology and to see how CPG can work with you. CPG is an ISO 17025 accredited contract research and analytical testing lab based out of Boston, MA.
[1] Gavin J. C. Braithwaite, Michael J. Daley & David Toledo-Velasquez (2016) Rheological and molecular weight comparisons of approved hyaluronic acid products – preliminary standards for establishing class III medical device equivalence, Journal of Biomaterials Science, Polymer Edition, 27:3, 235-246, DOI:10.1080/09205063.2015.1119035
 Vitrum Flexile, or flexible glass. Although lost in legend, three different authors provided an account of a glass that could be dented and then repaired in Rome’s first century. According to the Corning Museum of Glass, Petronius (who died in 63 A.D.) told of a drinking vessel presented to Emperor Tiberius (reign 14-37 A.D.). The vessel was thrown to the ground, and though dented, the glassmaker was able to remove the dent with a hammer.
Vitrum Flexile, or flexible glass. Although lost in legend, three different authors provided an account of a glass that could be dented and then repaired in Rome’s first century. According to the Corning Museum of Glass, Petronius (who died in 63 A.D.) told of a drinking vessel presented to Emperor Tiberius (reign 14-37 A.D.). The vessel was thrown to the ground, and though dented, the glassmaker was able to remove the dent with a hammer.
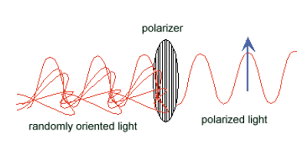 Figure 1: Schematic of randomly oriented white light passing through a polarizer, becoming plane-polarized light with the principle axis indicated by the blue arrow.
Figure 1: Schematic of randomly oriented white light passing through a polarizer, becoming plane-polarized light with the principle axis indicated by the blue arrow.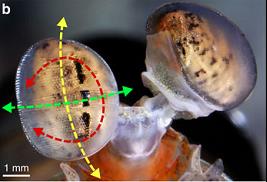 Figure 2: Rotational degrees of freedom in Odontodactylus cultrifer.
Figure 2: Rotational degrees of freedom in Odontodactylus cultrifer.

 At Cambridge Polymer Group, we help many of our clients evaluate their materials or device by applying accelerated aging techniques to accelerate material degradation, either for shelf life estimation or as part of an evaluation of material stability. Such testing may be performed following standard procedures like ASTM
At Cambridge Polymer Group, we help many of our clients evaluate their materials or device by applying accelerated aging techniques to accelerate material degradation, either for shelf life estimation or as part of an evaluation of material stability. Such testing may be performed following standard procedures like ASTM