TechConnect World Innovation
2024, June 17-19

CPG exhibited at TechConnect World, Booth 518, at Gaylord National Hotel & Convention Center, 201 Waterfront Street, National Harbor, MD.
72nd ASMS Conference on Mass Spectrometry & Allied Topics
June 2-6
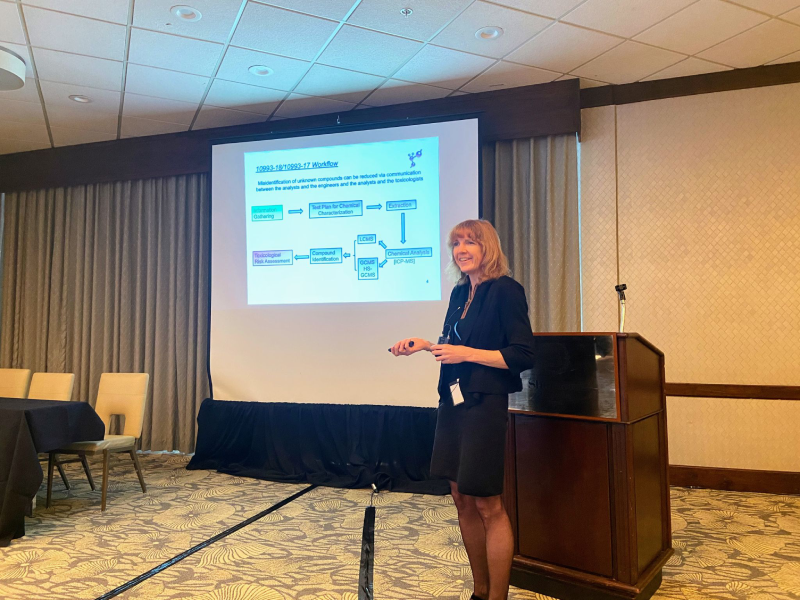
Dr. Michael Young, CPG Research Scientist, presented a poster at ASMS in Anaheim.
Smithers’ Extractables And Leachables USA
2024, May 21

Dr. Becky Bader, CPG’s Associate Director of Chromatography, spoke at Smithers’ Extractables and Leachables USA 2024 in Bethesda, MD on May 21 at 11:45 am, on “A Comparative Study of Extraction Techniques in E&L Testing.”
ASTM F04 Committee Workshop on Best Practices for Precision and Bias for Medical Device Standardization
2024, MAY 7

This workshop provided a forum to exchange best practices for developing precision and bias statements for medical device standards. Dr. Stephen Spiegelberg, CPG Founder,, co-chairs. 8:30 am to 12:00 pm, Philadelphia Marriott Downtown, Philadelphia, Pennsylvania. In-person only. Click here for more information.
SPE Mini Tech
2024, February 5-6
On Tuesday, February 6, Cambridge Polymer Group scientists presented two talks during the 1:30-3 p.m. Characterization & Validation of Medical Devices session:
- Particulate Testing for Additive Manufactured Medical Devices, Dr. Becky Bader
- Lab X Found WHAT In My Extracts? Best Practices in Unknowns Identification in Extractables Testing, Dr. Michael Young
Massachusetts Manufacturing Mashup
September 19
Worcester Polar Park at the FORGE tabletop exhibition space.
Cleaning Validation Summit 2022
December 1-2
CPG President Stephen Spiegelberg serves as a panelist at PharmaEd’s Cleaning Validation Summit 2022, December 1-2 in San Diego, California.
BIOMEDevice Boston
September 28-29

Stop by Booth 1120 at BIOMEDevice, Boston Convention & Exhibition Center, 415 Summer St., Boston.
Massachusetts Manufacturing Mash-Up
September 16
Join us at the 2nd Massachusetts Manufacturing Mash-Up!
FORGE – Material Selection for Physical Product Development
May 17
CPG President Stephen Spiegelberg participated on the FORGE panel about choosing materials wisely for increased product success in medical or harsh environments. UMass Lowell, 110 Canal Street, 3rd flr, Lowell. 5-6:30 p.m.
ARVO
May 1-4

VP of Research Gavin Braithwaite presents a poster on”
VP of Research Gavin Braithwaite presented a paper at ARVO 2022 on “A Temporary Hydrogel Vitreous Substitute in Support of Retinal Detachment Repairs” in Denver.
MDM West 2022
April 12-14

Massachusetts Manufacturing Mashup 2021
September 28

Cambridge Polymer Group exhibited at the Massachusetts Manufacturing Mash-Up, Polar Park, Worcester, MA on September 28.
MDM West 2021
August 10 -12
CPG Moves to Woburn
August 4 - 5
The 117% increase in lab space will allow us to meet the growing demand for our analytical testing and research & development services.
MIT Polymer Day 2021
April 28
Due to the ongoing COVID-19 pandemic, Polymer Day 2021 will be taking place in a completely virtual format.
Generis American Medical Device Summit, Booth 51
October 28-29, 2019
CPG VP Gavin Braithwaite presented A New Vision for Biomaterials: Crafting Biodegradable Hydrogels for Ophthalmic Devices:
- Discussing multi-discipline requirements for the development of soft-solid systems in biomedical applications
- Reviewing design processes for soft-solids from product requirements to final device
- How to track soft-solids in vivo
PharmaEd’s Cleaning Validation Summit 2019
October 9-10
CPG President Stephen Spiegelberg presented ASTM Standards in Medical Device Cleaning Validation at the PharmaEd Cleaning Validation Summit 2019.
Greentown Learn Manufacturing Initiative Supplier & Innovation Showcase
September 17

Cambridge Polymer Group sponsored Greentown Learn Manufacturing Initiative Supplier & Innovation Showcase.
ASTM Workshop on Accelerated Aging Methods and Testing Techniques for Medical Devices
May 14

CPG President Stephen Spiegelberg co-chaired an accelerated aging workshop, sponsored by ASTM Committee F04 on Medical and Surgical Materials and Devices, in Denver, Colorado.
CPG Research Scientist Norma Turner’s presented “Differentiating the effects of temperature and elevated oxygen pressure on accelerated aging of UHMWPE.”
AAPS PharmSci 360 2018, Booth 1343
November 4-7
AAPS PharmSci360, Walter Washington Convention Center, DC
AMI’s Polymer Testing & Analysis 2018
October 24-25
Cambridge Polymer Group attended the 2018 American Medical Device Summit on October 24-25 in Lombard.
PharmaEd’s Cleaning Validation Summit 2018
September 12-13
CPG talks at PharmaEd’s summit in Philadelphia:
Wednesday, September 12
CPG President Stephen Spiegelberg presented “ASTM Standards in Medical Device Cleaning Validation” at 10:40 a.m.
Thursday, September 13
CPG Research Scientist Adam Kozak presented “Development of Chromatography Assays For Cleaning Studies of Medical Devices” at 8:45 a.m.
AMI’s Polymer Testing & Analysis 2018
September 11-12

CPG Research Scientist Adam Kozak presented “Size (and Shape) Matter: Challenges and Strategies for Difficult Molecular Weight and Polymer Architecture Analysis” on September 11, Pittsburgh Marriott City Center, 112 Washington Pl, Pittsburgh, PA.
MDM East 2018
June 12-14

NPE 2018
May 7-11

Stop by Booth S30200 in the South Hall Level 1 – Expo Hall at NPE 2018: The Plastics Show, Orange County Convention Center, 9800 International Dr, Orlando. CPG Research Scientist Adam Kozak presents “Degradation Products of Medical Devices in Complex Biological Environments” on Wednesday, May 9, from 3:30-4 p.m. at the ANTEC Technical Program @NPE 2018.
BIOMEDevice 2018
April 18-19

MDM West
February 6-8, 2018

Extractables & Leachables Symposium for Drugs & Devices
October 12, 2017

CPG researcher Adam Kozak spoke at Eurofins’ Extractables & Leachables Symposium for Drugs & Devices in Lancaster, PA.
Cleaning Validation Summit
October 2-3, 2017
CPG President Stephen Spiegelberg spoke at PharmaEdResources’ Cleaning Validation Summit 2017 in Philadelphia on Tuesday, October 3.
American Pharma Outsourcing Summit 2017
September 27-28, 2017
CPG exhibited at the American Pharma Outsourcing Summit 2017, Doubletree by Hilton Boston North Shore, Danvers, Massachusetts.
AMI’s Polymer Testing & Analysis
September 19-20, 2017

CPG exhibited at Polymer Testing & Analysis at the Pittsburgh Marriott City Center.
Cambridge Polymer Group Awarded $225,000 SBIR Grant from the National Institutes of Health
August 14, 2017
Cambridge Polymer Group’s team of hydrogel scientists are developing a hydrogel that can be injected as a liquid through a fine needle or cannula, and then gels in the eye. This hydrogel will assist in keeping the retina attached to the back wall of the eye, and will then degrade over time, obviating the need for a second surgery. The hydrogel should also allow vision through the eye while it is in place, resulting in greater patient mobility and compliance.
MDM East 2017
June 13-15

CPG President Stephen Spiegelberg presented a workshop on Developing a Testing Plan for Medical Device Design Verification.
Minimizing Risk in Medical Device Material Selection
May 31, 2017

Dr. Brian Ralston discussed material selection considerations and process, selection of performance specifications, material screening, full assembly or sub-assembly prototype on candidate materials, and simulated testing of device. To view the webinar recording, click here.
UNH Bioengineering Symposium 2017
May 10

Dr. Gavin Braithwaite was an invited speaker and judge at the annual UNH Bioengineering Symposium. He presented “Designing Medical Devices Using Soft Materials.”
ANTEC/SPE 2017
May 8-10

CPG’s Dr. Brian Ralston presented “Medical Plastics: Where to Start with Material Selection” at ANTEC 2017.
BIOMEDEVICE 2017
May 3-4

Cambridge Polymer Group exhibited at BIOMEDevice, Boston Convention & Exposition Center.
CPG Exhibits at ORS, San Diego
March 20 - 22, 2017

CPG Receives ISO 17025:2005 Accreditation
March 2017
Cambridge Polymer Group is pleased to announce that the American Association for Laboratory Accreditation (A2LA) has approved our company for ISO 17025:2005 accreditation.
Cleanliness in Medical Devices Webinar
February 23, 2017

CPG’s webinar on cleanliness in medical devices included examples of what happens when cleaning processes are not properly verified and validated, how to establish the number of samples to test, how to test for device cleanliness, and how to establish acceptable residue limits.
Dr. Stephen Spiegelberg is the chairman of the ASTM task group on Medical Device Cleanliness, and Dr. Gavin Braithwaite regularly consults on cleaning issues in the medical device area.
To listen to a recorded version of the webinar, register here.
CPG Receives Patent for Patient Positioning
January 2017
CPG researchers, along with orthopedic surgeons from the Massachusetts General Hospital, have received a U.S. patent for a low cost navigation system designed to assist surgeons in aligning the acetabular cup used in hip replacement surgeries. The patent was issued as patent number 9,554,731.
CPG President Speaks at International Standards Meeting about Cleanliness in Medical Devices
January 24 -25, 2017

Scientists from the FDA, American Dental Association, medical device companies, and contract research laboratories were asked to present to Peruvian medical device regulators and manufacturers on how ASTM standards benefit their organizations in helping to get safe and effective medical devices into the patients who need them. Peru has a keen interest in improving their adoption of standards for material and product testing in order to market safe products both in Peru and outside of Peru.
Dr. Stephen Spiegelberg from Cambridge Polymer Group spoke about his ASTM task group on Medical Device Cleanliness, and provided examples of what can go right and what can go wrong when ASTM standards are not properly used, particularly with respect to validation of medical device cleaning lines.
Read more in this ANSI article and this ADA article.
MRS Fall Meeting & Exhibit
November 29 - December 1, 2016

Cambridge Polymer Group exhibited at the 2016 Material Research Society Fall Meeting & Exhibit.
CPG ACHIEVES ISO 9001:2015 CERTIFICATION
November 1, 2016

In November 2016, CPG successfully transitioned from ISO 9001:2008 to the updated ISO 9001:2015 standard. Read more about CPG’s new ISO certification.
FDA Workshop on Refurbishing of Medical Devices Performed by Third-Party Entities and Original Equipment Manufacturers
October 28, 2016
CPG President Stephen Spiegelberg spoke at an FDA workshop on “Refurbishing, Reconditioning, Rebuilding, Remarketing, Remanufacturing, and Servicing of Medical Devices Performed by Third-Party Entities and Original Equipment Manufacturers” in Silver Springs, Maryland.
MD&M East
June 14-16, 2016

Cambridge Polymer Group exhibited at MD&M East, the Javits Convention Center, NYC, Booth 175. For more info, click here.
World Biomaterials Congress
May 17-22, 2016

CPG researcher Adam Kozak presented a poster on a cardiovascular device designed to reduce mitral valve regurgitation following a ventricular infarction. For more info on the World Biomaterials Congress in Montreal, click here.
CPG Exhibits at BIOMEDevice 2016
April 13-14, 2016

CPG Sponsors MIT Polymer Day
March 30, 2016

Cambridge Polymer Group sponsored MIT’s 6th Annual Polymer Day on March 30th, 2016. CPG scientists Gavin Braithwaite and Brian Ralston were judges at the poster contest, where MIT researchers presented their latest research. For more on MIT’s Polymer Day, click here.
Patent on Mosaicplasty Implants Issued to CPG Researchers
March 22, 2016
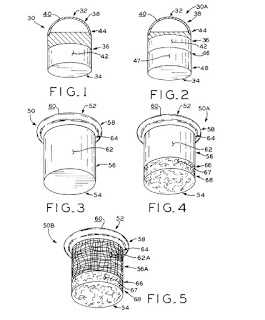
US Patent 9,289,302 was issued on March 22, 2016 to CPG researchers. The patent describes designs and methods for making implants for focal cartilage tears in load bearing implants, such as hips and knees. The construct designs make use of a surgical procedure termed mosaicplasty, whereby a plug of healthy osteochondral plug is removed from a non-load bearing portion of the joint, and placed in a drilled out cavity in the focal defect. In this patent, synthetic plugs are created based on hybrid structures of polymers and metal repair the focal defects.
CPG Exhibits at ORS 2016 Orlando
March 5-8, 2016

Notch Fatigue in UHMWPE
March 2016

CPG researcher Adam Kozak co-authored a Journal of Mechanical Behavior of Biomedical Materials article. Along with co-authors from UC-Berkeley (Ansari, Gludovatz, Ritchie, and Pruitt), these researchers investigated the sensitivity of ultra high molecular weight polyethylene (UHMWPE) to fatigue when stress concentration sites are present in the form of notches. The authors investigated 3 formulations of UHMWPE commonly used in hip or knee implants today, and found that the sensitivity to crack propagation resulting from fatigue was more sensitive to microstructure, such as crosslink density, rather than specifics of the notch geometry.
The Rheology of Hyaluronic Acid Used to Treat Osteoarthritis
January 2016

Recently researchers at Cambridge Polymer Group in collaboration with OrthogenRx coauthored a paper on biosimilar medical devices and methods for supporting performance similarities in specifically viscosupplementation. In this paper, we compare an existing material cleared for sale in the US, against an ex-US device intended to replicate the performance of the existing material as a “generic device” equivalent to generic drugs. The ability to market generic devices has only recently been made possible through changes in the regulatory mechanisms in the US, and this paper discusses this context and describes analytical methods for comparing the performance of viscosupplementation materials. Read the paper here.
CPG Acquires Herzan AVI-400 for SEM
January 2016
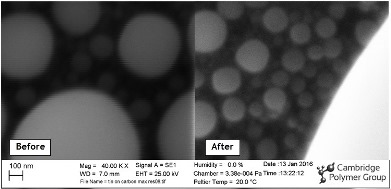
Over two years ago, CPG purchased a Zeiss EVO LS15 environmental scanning electron microscope. This instrument has become a work-horse for root-cause analysis, surface topographies and (through the attached Oxford EDS) elemental mapping. With the large chamber and capability to run in “wet mode” (hydrated at elevated pressures) it has also proved invaluable at imaging hydrogel structures and friable materials such as foods and tissues.
As part of an effort to improve the resolution of the instrument, CPG recently procured a Herzan AVI-400 antivibration solution. The entire SEM system sits on this active vibration damping system, removing virtually all of the building and environmental vibration. We are delighted that the Zeiss now achieves resolutions close to the ideal specifications of the instrument.
CPG Exhibits at MRS 2015 Boston
December 1-3, 2015

3rd Edition of UHMWPE Biomaterials Handbook
October 27, 2015

This edition contains the history of ultra high molecular weight polyethylene and its use in hip and knee arthroplasty. The new edition contains multiple chapters addressing analytical testing techniques to characterize UHMWPE, wear testing, accelerated aging, antioxidant effects, and advances in UHMWPE processing and formulation development.
CPG researchers Braithwaite, Kozak, and Spiegelberg contributed a chapter on characterization techniques on UHMWPE, including details on fatigue crack propagation testing, true stress-true strain measurements, and electron spin resonance spectroscopy.
Fatigue Crack Propagation Testing in UHMWPE
October 22, 2015
This poster presentation discusses the comparison of fatigue crack propagation testing per ASTM E647 using a new optical system developed at Cambridge Polymer Group to monitor crack growth in an automated manner. The presentation shows results from two laboratories using the system. Presented at the 7th semi-annual UHMWPE Conference on October 22, 2015 in Philadelphia, PA.
Medical Grade Polymers 2015
September 15-16, 2015

Dr. Yuri Svirkin, CPG Research Scientist, and Mr. Adam Kozak, CPG Research Scientist, presented “Novel Material Development for Medical Devices.”
MIT Celebrates Polymer Day
March 11, 2015

On March 11, MIT’s Program in Polymers and Soft Matter hosted their 5th Annual Polymer Day. A poster session was held in the morning, and two CPG scientists, Dr. Yuri Svirkin and Adam Kozak, acted as judges and awarded prizes to the top posters.

Cambridge Polymer Group received an appreciation award for three years as a Polymer Day Sponsor. Dr.Yuri Svirkin (left) and Research Scientist Adam Kozak (center) accepted the award.
CPG Exhibits at 2014 MRS Fall Meeting & Exhibit
December 2-4, 2014

MD&M Chicago
October 16, 2014

CPG researcher Stephen Spiegelberg presented “Failure Analysis of Medical Plastics.”
CPG Acquires Variable Pressure SEM
August 2014
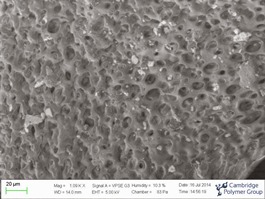
CPG recently acquired a variable pressure scanning electron microscope (SEM). This system is useful for failure analysis of components, in that the imaging is non-destructive. Additionally, the CPG system has a cold stage, which allows the control of relative humidity and temperature in the chamber, which is useful for imaging water-containing samples such as tissue and hydrogels. The system acquired also has a large capacity chamber enabling in most cases visualization on entire devices and components. More info
CPG Acquires an ESR for Analysis of Free Radicals
June 2014
Electron Spin Resonance is used to determine concentrations of free radicals in foods and materials. In the beer industry it is invaluable as a measure of how prone the beer is to “staling” where beer-derived free-radicals ultimately result in the production of carbonyl end products that result in the distinctive “off” flavor of old beer. In the orthopaedic industry free radicals are induced by the crosslinking, or other manufacturing process, and therefore tracking these radicals is critical for determining ultimate stability of the materials. CPG’s acquisition of an ESR, along with the extensive calibration and validation we have performed, provides a valuable tool in determining likely stability of materials.
CPG Exhibits at Medical Device and Manucturing East
June 10-12, 2014

US Patent Issued to CPG
May 20, 2014

CPG was issued US Patent 8728379 in May 2014. This patent describes methods of making wear resistance, oxidatively stable polyethylene for orthopedic implants. The technology involves irradiating ultra high molecular weight polyethylene (UHMWPE) which contains Vitamin E as an antioxidant. This patent is available for license. For more information, please visit our web site.
CPG Publishes Journal Article on Vitamin E Measurement Technique for UHMWPE
April 28, 2014

CPG researchers recently published a paper on a sensitive technique to quantify Vitamin E in UHMWPE used for orthopedic applications. The article is published in the Journal of Biomedical Materials Research.
CPG Receives Patent for Highly Lubricious and Tough Hydrogels
January 28, 2014

CPG inventors were assigned US patent 8,637,063 for methods of making highly lubricious hydrogels that may be suitable for joint replacement or bearing surfaces.
These hydrogels are manufactured in a novel manner by first forming an organogel, and then converting this organogel to a hydrogel. This technology is available for license, learn more here.
CPG Acquires a TGA with FTIR Analysis Accessory
January 2014
CPG recently acquired a thermal gravimetric analyzer. This tool provides the ability to separate compounds from a material by their evaporation point, and also examination activation energies and ash content. In conjunction with the FTIR accessory, we can also determine the molecules begin involved. This combination of tools expands our ability to perform deformulation work, particularly in complex polymeric systems. Using the TGA in a “pyrolysis” mode allows identification of individual polymeric species, as well as additives in the final composition.
CPG Receives Patent for Gradient Antioxidant UHMWPE
October 29, 2013

CPG inventors, along with inventors from the Massachusetts General Hospital, were assigned US Patent 8,569,395 for methods of making oxidation-resistant crosslinked polymeric materials. This patent discusses methods of incorporating antioxidants into UHMWPE to allow spatial control of crosslinking and antioxidant distribution, and has applications for total hip, knee, and shoulder arthroplasties. This technology is available for license. Learn more here.
Fall MRS Research Presentation
2013
CPG researchers Yuri Svirkin, Adam Kozak, and Gavin Braithwaite presented their work on thiol-modified PVA hydrogels at the Fall Materials Research Society Meeting in Boston on December 3rd (Paper E5.09). Learn more about 2013 MRS Fall Meeting here.
2013 (Houston, TX). MedTech Polymers Conference
October 15-16

Dr. Stephen Spiegelberg presented a paper on failure analysis of medical devices, including testing methods. More Info
CPG aquires new Impact Tester
October 2013

The recently purchased CEAST 9050 (Instron) impact tester can perform Izod testing in compliance with both ASTM D256 and UHMWPE testing per ASTM F648.
CPG Scientist Elected to post in ASTM committee
September 2013

CPG researcher Dr. Stephen Spiegelberg was recently elected to the post of Recording Secretary for ASTM Committee F04 “Medical and Surgical Materials and Devices”. Click here for more information on the committee.
Cambridge Polymer Group announces the opening of a new West Coast office
August 2013

Cambridge Polymer Group has expanded its operation with the opening of a West Coast office to support growing demand for materials consultation. Office manager: Ayyana Chakravartula, PhD. (617) 629-4400 Ext. 23.
US Patent for High Crosslinked UHMWPE Issues
August 22, 2013
US Patent 8530057 “Methods for making oxidation resistance polymeric material” issued this year. This patent discusses methods of making highly crosslinked ultra high molecular weight polyethylene (UHMWPE) resistant to oxidation by incorporating vitamin E, a naturally occuring antioxidant. This material is available for licensing, and has been cleared by the FDA and notified bodies in the EU for use in hip and knee devices.
Polymers and Plastics in Medical Devices Conference (San Francisco, CA)
June 26-28, 2013
CPG researcher Gavin Braithwaite presented a paper on hydrogel use in medical devices. The paper discusses the design of cartilage replacement technologies using hydrogels.
Australian Patent for Highly Crosslinked UHMWPE Issues
May 30, 2013
Australian patent 2010206016 “Methods for making oxidation resistance polymeric material” issued this year. This patent discusses methods of making highly crosslinked ultra high molecular weight polyethylene (UHMWPE) resistant to oxidation by incorporating vitamin E, a naturally occuring antioxidant. This material is available for licensing, and has been cleared by the FDA and notified bodies in the EU for use in hip and knee devices.
Program in Polymer Science and Technology – Polymer Day
March 8, 2013
MIT held its annual MIT Polymer Day in March. Cambridge Polymer Group was a sponsor, and also supplied two judges for the poster contest, where students and post-doctoral research fellows presented their work on polymer technology. More information.