With the upcoming ASTM workshop on reprocessing of re-usable devices, we thought a primer on this important area of the health care industry would be in order. Reprocessing is a set of procedures that will take a previously used medical device and return it to a state fit for a subsequent clinical use. Usually, the most important steps in reprocessing include (1) cleaning; and (2) disinfection or sterilization.
Cleaning
The cleaning step should remove biological soils, such as blood and tissue, as well as any other materials that may have contacted the medical device, such as hospital disinfectants, lubricants.
Disinfection
The disinfection or sterilization step is a completely separate step from cleaning, and neither can be substituted for the other. The choice of disinfection (the killing of some or most microorganisms, with the exception of bacterial spores, depending on the level of disinfection) or sterilization (killing all microorganisms to a log reduction, typically 1E-6) depends on the application area where the medical device is used.
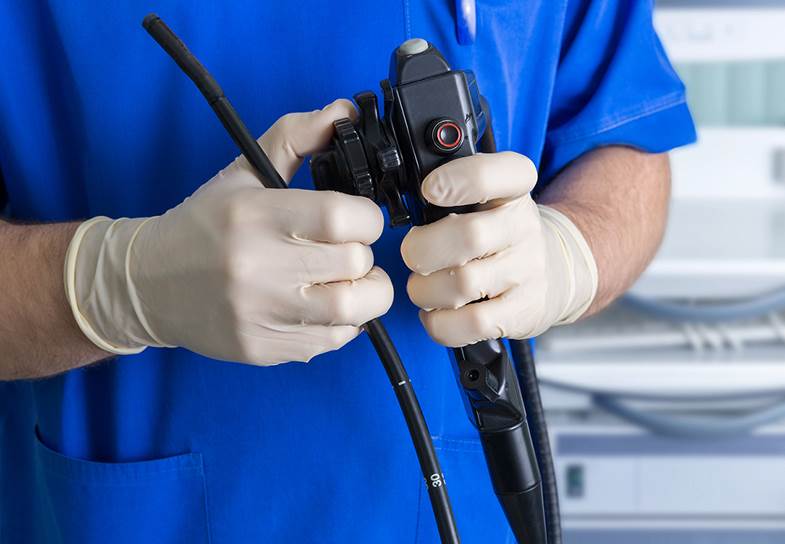
Re-usable Medical Devices
A re-usable medical device is one that is intended to be used on multiple patients with reprocessing between each patient. Examples of reusable devices include forceps, stethoscopes, endoscopes, scissors, arthroscopic shavers, and suction tubes. Reprocessing is often conducted either at hospitals or by third body reprocessors. It is up the reprocessor to demonstrate a validated reprocessing procedure for each medical device, which involves demonstration of adequate cleaning through verification tests. The original equipment manufacturer should ensure that the device is designed and constructed to allow reprocessing, and provide adequate instructions on how to reprocess the device, along with appropriate labeling. Proper selection of materials that can undergo repeated reprocessing is an important consideration as well.
The FDA issued a guideline on reprocessing of re-usable medical devices in 2015.