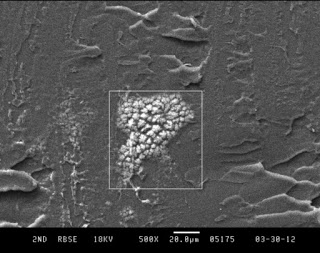
Fingerprint Analysis via SEM
Analysis of fracture surfaces of samples is sometimes complicated by inadvertent contamination the the sample surfaces during sample preparation. Dust and other debris from the lab bench, in addition to particles generated from cutting instruments, can contaminate an otherwise clean surface. If gloves are not worn during sample preparation, finger oils and salts can be […]
Accelerated Aging
Accelerated aging protocols are key to medical device development. For initial product design, manufacturers of medical devices would like to know how their devices will respond to an in vivo environment, for a time period that could extend to decades in the case of permanent implants. For product labeling and regulatory, the manufacturer needs to […]

Implants in the After Life
Ever wonder what happens to metal hip and knee replacements after their recipient expires? How about metal pins and screws to hold bones together? In the past, these components, often composed of expensive metals such as cobalt chrome, tantalum, and titanium, were buried with the patient. Now, a Dutch Company, OrthoMetals, has teamed up with […]
 Collagen powder isolated on white background. with clipping path
Collagen powder isolated on white background. with clipping path
Crush Strength of Catalyst Material
Catalyst powder for chemical reactions is often formed into a packed bed and placed into a reactor vessel. In packing the catalyst, the formation of smaller particles, or fines, can occur if the packing pressure exceeds a critical value. This fine formation is undesirable, as it increases the potential for bed compaction and subsequent increase […]
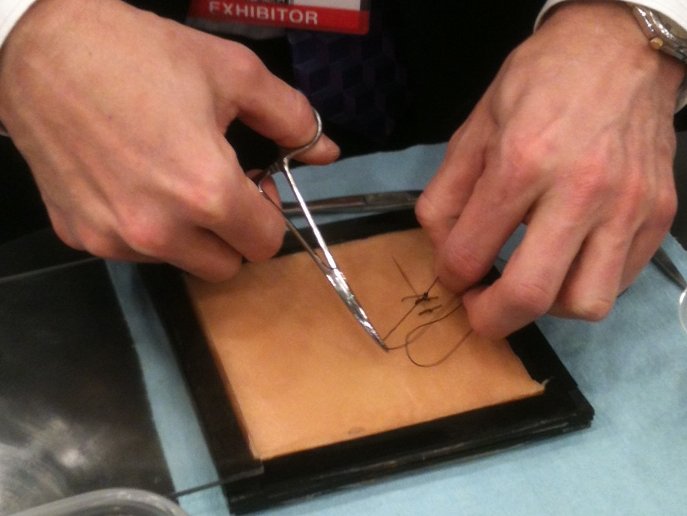
Tissue Block for Suture Practice
Traditionally, medical students have practiced suturing on tissue mimics made from silicone or polyurethane elastomers. These materials lack the lubriscious nature of natural tissue. Using our proprietary hydrogel technology, CPG has developed single and multi-layer tissue blocks that contain a similar amount of water as natural tissue, and hence provides a similar feel as natural […]
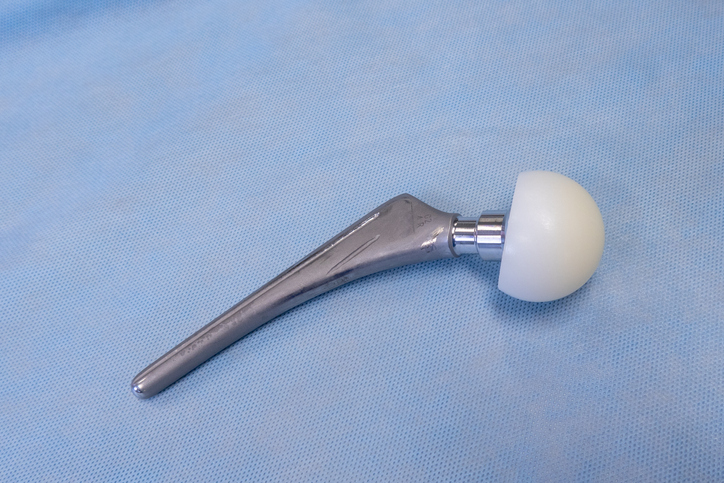 explanted surgical hip prosthesis lies on an operating table
explanted surgical hip prosthesis lies on an operating table
FDA Clears Ecima
The FDA has cleared ECiMA(tm), a highly crosslinked polyethylene containing Vitamin E, for use in hip arthroplasties. ECiMA is sold by Corin, and was developed by researchers at Cambridge Polymer Group and the Massachusetts General Hospital. ECiMA was developed as a second generation highly crosslinked UHMWPE to replicate the good wear properties of the first […]
Hip Implant Recall
Johnson & Johnson has continued to investigate their metal-on-metal implants, which were recalled in 2010 due to some patients reactions to metal debris generated during articulation. In a Reuter’s report today, J&J had fourth quarter charges of $800 million associated with medical costs related to the recall.

Radiopacity in Medical Devices
Temporary or permanent implants often contain a radiopacifier, which is a material with a higher electron density contrast compared to the surrounding material so that it absorbs X-ray energy. In an X-ray, a radiopacifier appears as a bright section, as shown in the catheter above (the internal wire is a radiopacifier). Radiopacifiers are often made […]
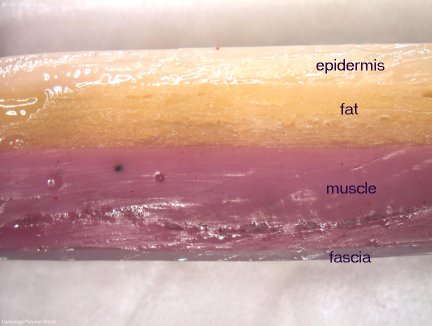
Hydrogel Skin Model
Synthetic tissue constructs have been around since the 1970’s, when Dr.’s Yannas and Burke created an artificial skin from collagen and silicone rubber. This membrane, termed Silastic, was designed to mimic the properties of skin, to help generate new skin in burn victims. Researchers from the Medical School Hannover (Germany) are trying to replicate human […]
Highly Crosslinked UHMWPE Available for License
Cambridge Polymer Group and Massachusetts General Hospital have co-developed novel, highly crosslinked ultra high molecular weight polyethylenes that incorporate vitamin E and are suitable for hip, knee, shoulder and spine arthroplasty applications. These technologies, generically termed CIMA, E-CIMA and Reservoir Vitamin E, are available for license. E-CIMA E-CIMA is a formulation containing Vitamin E throughout […]
