
ASTM Workshop on Additives in Biomedical Polymers: Call for Papers
Committee F04 is seeking abstracts for a workshop on Additives in Biomedical Polymers, to be held on May 6, 2014 in Toronto, Ontario. This workshop is intended to elicit discussion on the benefits, potential hazards, testing, regulatory considerations, and opportunites for standards activities related to intentionally incorporated additives in biomedical polymers. Information on how to […]
Presentation on Biodegradable Thiol-Modified Polyvinyl Alcohol Hydrogels
CPG researchers Yuri Svirkin, Adam Kozak, and Gavin Braithwaite will be presenting their work on thiol-modified PVA hydrogels at the Fall Materials Research Society Meeting in Boston at 4:45 pm on December 3rd (Paper E5.09). Abstract Poly(vinyl alcohol) (PVA) is a well-respected biomaterial and forms highly hydrated hydrogels. It has been used in a number […]

How Dense is that?
Density, or the ratio of the mass of an object to its volume, is a commonly reported material parameter. Density is influenced by the chemical composition of the material, crystallinity, and porosity. The chemical composition depends on the elements that make up the material. Plastics are normally composed of hydrocarbons (carbon and hydrogen), which have […]
Updates from the November F04 ASTM Meeting
The medical device task group of ASTM met in Jacksonville, FL from November 12-15th. The meeting starting with a workshop on modularity and tapers in total joint replacement devices, and discussed potential issues with femoral head fretting. The cleanliness task group worked on two draft standards. The first, a guideline for validation of medical device […]
Molecular Weight Distribution From Rheology
The molecular weight distribution of polymers strongly influence their properties, such as tensile strength, crack resistance, and solubility. Gel permeation chromatography is a commonly used technique to measure molecular weight distribution, but relies on the ability of the polymers to be dissolved in a solvent that is readily usable in a GPC column. Additionally, the […]

Making an Impact
CPG recently purchased a new impact tester, a CEAST 9050 (Instron). With both V-notching and blade notching capabilities, we can perform Izod impact testing on materials in compliance with ASTM D256, as well as impact testing on UHMWPE per ASTM F648. The pneumatic release option on the impact tester allows very reproducible results.

CPG Scientist Elected to a Post at ASTM
CPG researcher Dr. Stephen Spiegelberg was recently elected to the post of Recording Secretary for ASTM Committee F04 “Medical and Surgical Materials and Devices”. Click here for more information on the committee. The committee’s 160 members meet biannually, attending over two days of technical meetings capped by a symposium or workshop on relevant topics in […]
Visit CPG at the Medical Grade Polymer 2013 Technical Conference
17-18 September 2013 Crowne Plaza, Boston/Woburn, Massachusetts Click here for more information Stop by booth 10 and take a look at what we’ve been working on!Cambridge Polymer Group is your premier contract research resource solving problems with our multi-disciplinary research team and full service laboratory. We provide routine analytical testing on materials, custom test design, […]
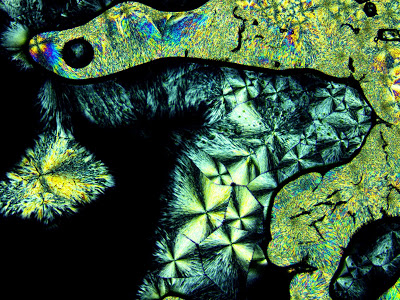
Birefringence in Crystalline Polymers
Polarized light microscopy is an effective tool to examine the crystalline structure of materials. In this technique, a sample is placed between two polarizers which are oriented 90 degrees to each other, or are “crossed”. Light is transmitted through the polarizers and samples into an objective. Light travelling through the first polarizer becomes polarized in […]

CPG Opens West Coast Office
August 2013 – Cambridge Polymer Group Announces The Opening Of A New West Coast Office Cambridge Polymer Group has expanded its operation with the opening of a West Coast office to support growing demand for materials consultation. The new office will be run by Ayyana Chakravartula, PhD. (617) 629-4400 Ext. 23, and is located in […]
