
Quantifying Rapid Rheological Changes
Blood vessel with hemostatic agent In the world of material science and product design, understanding and measuring rapid rheological transitions is crucial. These transitions, which can be triggered by various stimuli such as UV light, electrical fields, or chemical interactions, play a significant role in applications ranging from hemostatic agents to 3D printing materials. While […]
FDA’s Regulatory Freeze: Implications for Medical Device Standards and Patient Safety
Medical device development relies heavily on standards to ensure patient safety, efficacy, and regulatory compliance. Organizations such as ASTM, AAMI/ISO, and USP establish critical test methods to ensure adequate cleaning and sterility, mechanical performance, biocompatibility, and material integrity. These standards streamline innovation while safeguarding patients, reducing redundant testing, and maintaining U.S. competitiveness in global markets. […]

NAMSA Acquisition of U.S. Medical Device Testing Operations of WuXi AppTec
NAMSA, a medical device contract research organization, announced last week the acquisition of the US medical device testing operations of WuXi AppTec, a biopharmaceutical and medical device testing laboratory headquartered in China. This acquisition is part of a recent trend of acquisitions of medical device testing laboratories by larger multinational testing conglomerates over the past […]
INEOS ABS Closure: Impact on Medical Device Manufacturers
The recent announcement of INEOS’s decision to permanently close its ABS (acrylonitrile butadiene styrene) production facility in Addyston, Ohio, has sent ripples through the medical device manufacturing industry. This closure, set to begin in the second quarter of 2025, will significantly impact manufacturers who rely on ABS plastics for various medical applications. The situation calls […]
Have an ABS-olutely Fab Holiday
Holiday Hours Please note that our lab will be closed on the following dates to allow our team to enjoy the holidays: December 24 – 25 (Christmas Eve & Day) December 31 – January 1 (New Year’s Eve & Day) We will resume normal business hours on January 2nd, ready to tackle your new materials challenges in […]

New Release of ASTM F2459: Expanding Standards for Medical Device Cleanliness
ASTM recently published a new version of F2459 “Standard Test Method for Extracting Residue from Medical Components and Quantifying via Gravimetric Analysis.” This updated standard expands its scope beyond metallic implants to include ceramic and polymeric medical devices. Key Updates and Applications Expanded Scope: The standard now covers residue assessment for metallic, ceramic, and polymeric […]
Polymer Deformulation by Multi-Step Pyrolysis GC-MS
Polymer deformulation typically involves multiple tests to identify the type of polymer, assess the presence of potential blended polymers, and evaluate the incorporation of additives such as colorants, stabilizers, and processing aids. An elegant and more rapid alternative involves a form of pyrolysis gas chromatography mass spectrometry. During pyrolysis, polymeric materials are rapidly heated to […]
FDA Workshop Explores Potential Expansion of ASCA Program to Include Chemical Characterization
On November 6, 2024, the FDA held a workshop to discuss the potential expansion of the Accreditation Scheme for Conformity Assessment (ASCA) program to include chemical characterization per ISO 10993-18. This expansion would build upon the current ASCA program, which primarily covers select biological endpoint tests according to ISO 10993. Because the FDA has already […]
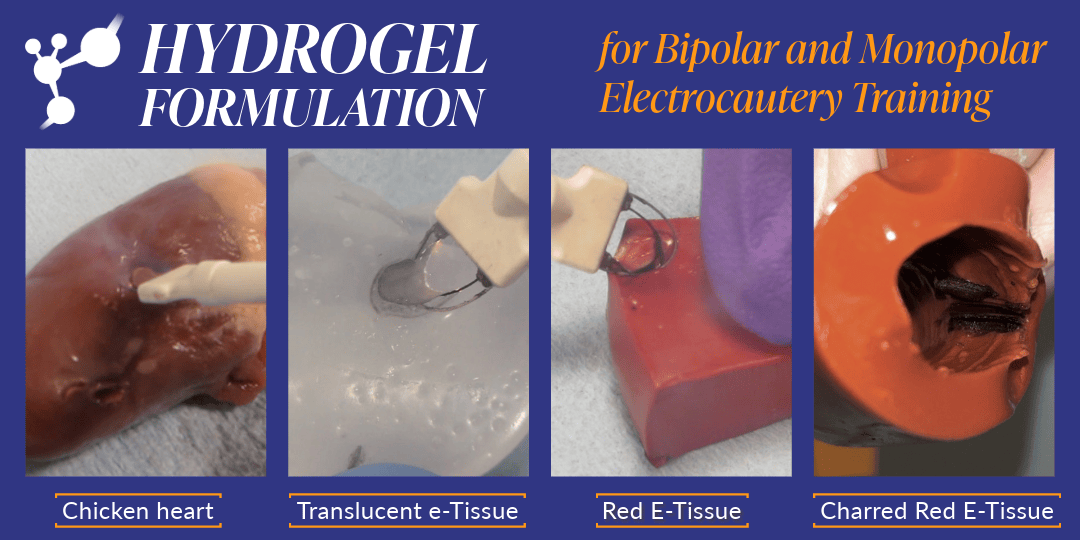
Improving Surgical Training with Custom Hydrogel Models
At Cambridge Polymer Group, we are constantly pushing the boundaries of polymer science to develop innovative solutions for our clients. Our recent advancement in hydrogel technology aims to enhance surgical training by offering realistic tissue phantoms that more closely mimic the behavior of human tissue during electrosurgical procedures. The Challenge: Realistic Tissue Simulation As the […]
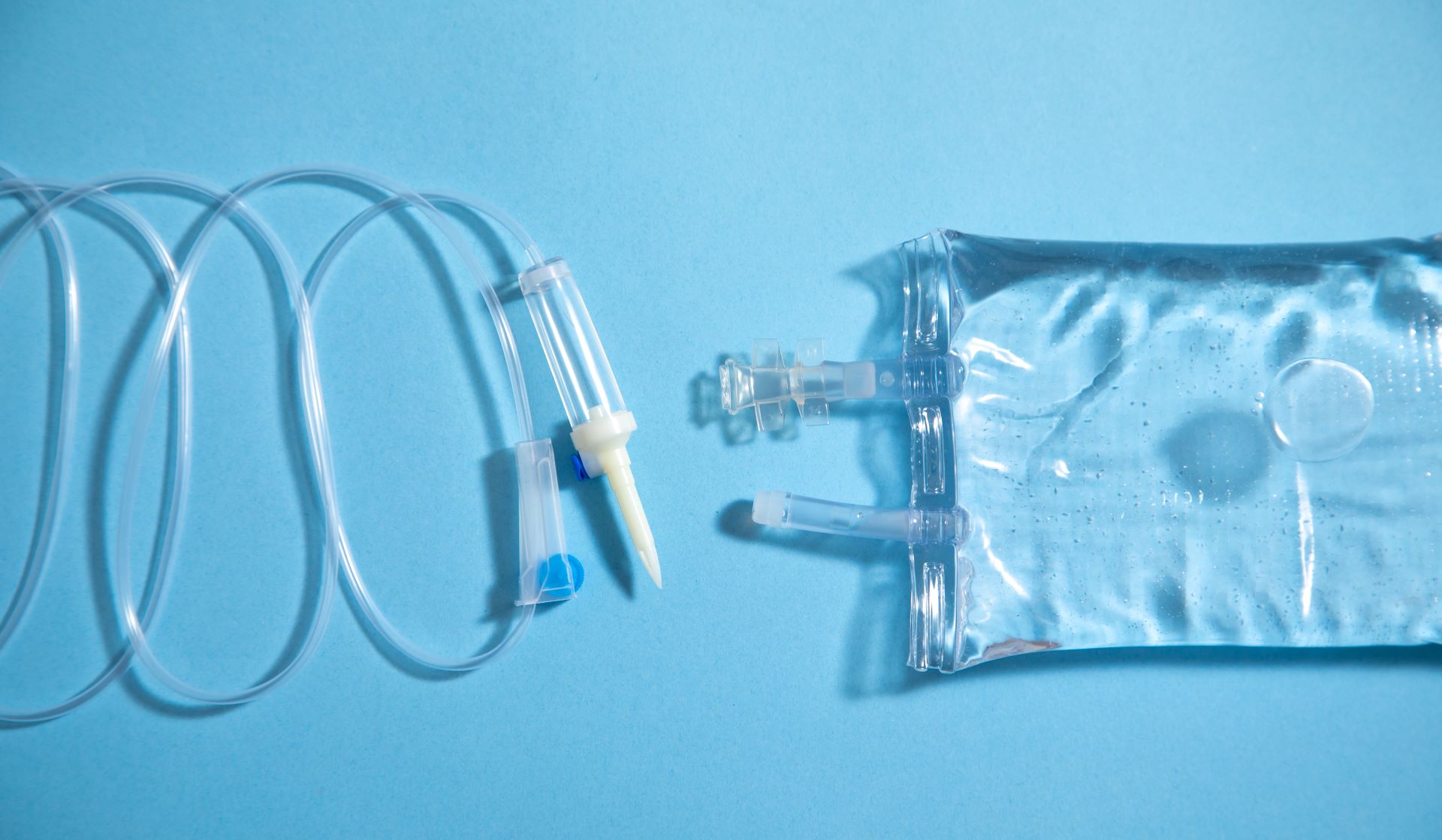
The California Phthalate Ban: A New Era For Medical Devices
California, known for its progressive stance on environmental and health issues, is once again at the forefront of regulatory change. On September 25, 2024, California Governor Gavin Newsom signed the Toxic-Free Medical Devices Act (AB 2300) into law. The legislation prohibits the use of certain ortho-phthalates, specifically di-(2-ethylhexyl) phthalate (DEHP), in intravenous (IV) solution containers […]
