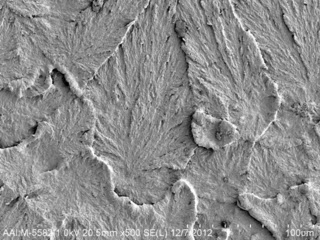
Consolidation Defects in UHMWPE
Ultra high molecular weight polyethylene (UHMWPE) is the most commonly used bearing surface in hip and knee arthroplasties. Due to its high molecular weight, UHMWPE cannot be injection molded or extruded with a screw extruder. Compression molding or ram extrusion are the two consolidation processes used for UHMWPE, whereby the combination of temperature and pressure […]
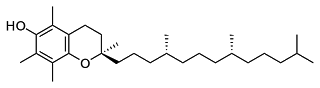
Analysis of Vitamin E Residues
The increasing prevalence of Vitamin E, a naturally-occuring antioxidant, in medical grade plastics has resulted in a need for analysis methods that can track the effect of processing on this compound. Vitamin E is effective as an antioxidant due to the hydroxyl group sitting on the aromatic ring at one end of the molecule. This […]

ASTM Meeting on Medical Plastics
The ASTM Committee on Medical Devices (F04) met last week in Atlanta, GA. Cambridge Polymer Group staff attended several of the task groups, and reported back the following activities. Medical Device Cleanliness Several draft standards are in development, including guidance on cleanline validation, synthetic test soils to verify cleaning efficacy, guidance on how to design […]
 Chocolate cookies biscuit dipped with a glass of milk.
Chocolate cookies biscuit dipped with a glass of milk.
How to Measure Volume
Measuring the volume of a standard shape (e.g. cube, cylinder, sphere) is straightforward, as one only needs to measure the relevant dimensions (length, height, diameter, etc.) and calculate the volume using known geometric equations. Measuring a non-standard shape is also straightforward if you have an analytical balance. Using Archimedes’ principle of buoyancy, the weight of […]

DJO Announces Plans to Launch Vitamin E Polyethylene Knee Implant
DJO Global announced that it will be launching its Motivation(tm) total knee system at the American Academy of Orthopedic Surgeons meeting in 2013. The Motivation System incorporates e+(tm), an UHMWPE blended with Vitamin E. Source: DJO
FDA Regulation of New UHMWPE Components
Dr. Michael Kasser, from Center for Devices and Radiological Health at the FDA, has published an article in the Journal of Biomedical Material Research detailing the history of regulation on ultra high molecular weight polyethylene (UHMWPE) and its use in medical devices. This article is available as a early view download for registered users. Dr. […]
Iconacy Orthopedic Implants Gets FDA Clearance on Cima
Iconacy Orthopedic Implants, a privately held medical device company in Warsaw, IN, received FDA clearance to market their highly crosslinked UHMWPE prepared using the CIMA process developed at Cambridge Polymer Group and the Massachusetts General Hospital. CIMA is a patented highly crosslinked, low wear and oxidation-resistant ultra high molecular weight polyethylene that is non-exclusively licensed […]

Mooney Rivlin Testing
The Mooney-Rivlin model is a hyperelastic model that can be used to predict the deformation behavior of elastomers to uniaxial, planar, and biaxial extension. This model requires knowledge of two or more Mooney-Rivlin parameters that are specific to the material in question, and usually to the deformation mode in question as well. Some experimental work […]
Nano-Patterning on Hip Replacements
The BBC reports that a team from Glasgow University has developed a technique to created a pitted nanopatterned surface using poly ether ether ketone (PEEK). Tissue growth studies conducted by this group has shown that the nanopattern helps to elicit bone growth through the manipulation of stem cells. These researchers feel that it they can […]
Presentation on Biomedical Applications of Hydrogels
The current standard of care in orthopedic joint replacement relies on the use of hard bearing surfaces comprised of polyethylene, ceramics, and metals. The natural tissues these synthetic materials replace are usually softer, viscoelastic materials that are best described as hydrogels, or hydrophilic network structures of cross linked macromolecules. In this audio conference presentation, our […]
