Webinar – October 15, 2025, 2 p.m. ET
Ensuring medical device safety requires a coordinated approach across three critical domains: biocompatibility, cleanliness, and sterility. While each area has its own regulatory and testing requirements, they are deeply interconnected. Overlooking the relationship between these elements can increase patient risk and delay product approvals. This upcoming medical device webinar explores how cleaning validation influences biocompatibility outcomes and overall product safety.
Cleaning validation is a foundational step in establishing device safety. Surface contaminants left from manufacturing or cleaning processes can directly impact biological evaluation results and compromise sterility assurance. Similarly, certain sterilization methods can alter material chemistry, generating new extractables or leachables that affect the device’s biocompatibility profile.
When designing and validating a medical device, both manufacturers and regulators should consider:
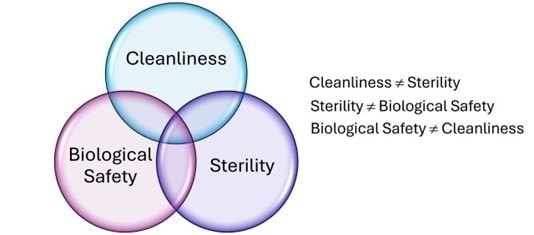
- Cleanliness addresses surface residues, which can directly influence biological evaluation results. It’s also essential to achieving reliable sterilization assurance.
- A sterile medical device may not necessarily be biocompatible; sterilization methods can alter chemical properties relevant to biological safety.
- Devices can meet cleaning and sterility standards but still fail biocompatibility due to material selection or design factors.
- Even if a device is determined to be biocompatible post-manufacture, poor cleaning or sterility assurance can compromise clinical performance and patient safety.
Join our webinar, From Residues to Risk: The Role of Cleaning Validation in Biocompatibility Assessments, on October 15, 2025, 2 p.m. ET, to learn how cleanliness, sterilization, and biocompatibility testing intersect during medical device validation. Our experts will highlight best practices for integrating cleaning validation into biocompatibility assessment plans, review regulatory expectations, and provide actionable strategies to minimize risk while ensuring compliance with ISO 10993 and related standards.
Register for free today via the link here.