Tentative Identifications in Medical Device Chemical Characterization
Analytical chemical characterization and toxicological risk assessment are essential for evaluating the risk posed by chemicals that may be present in medical devices. As detailed by ISO10993-17 and ISO 10993-18, this process can support the biological safety of a device through assessment of the toxicological risk of extractables and leachables.
The Role of Mass Spectral Expertise in Identification
In practice, the burden of identification of these chemicals relies heavily on mass spectrometry and the expertise of analytical chemists, without guidance from toxicologists. Confidence levels are assigned to each analyte based on the quality of the spectral data and the availability of reference standards. These confidence levels range from confirmed identifications to unknown, along with tentative or partial assignments, reflecting the real-world complexity of chemical analysis.
The Reality of Tentative Identifications
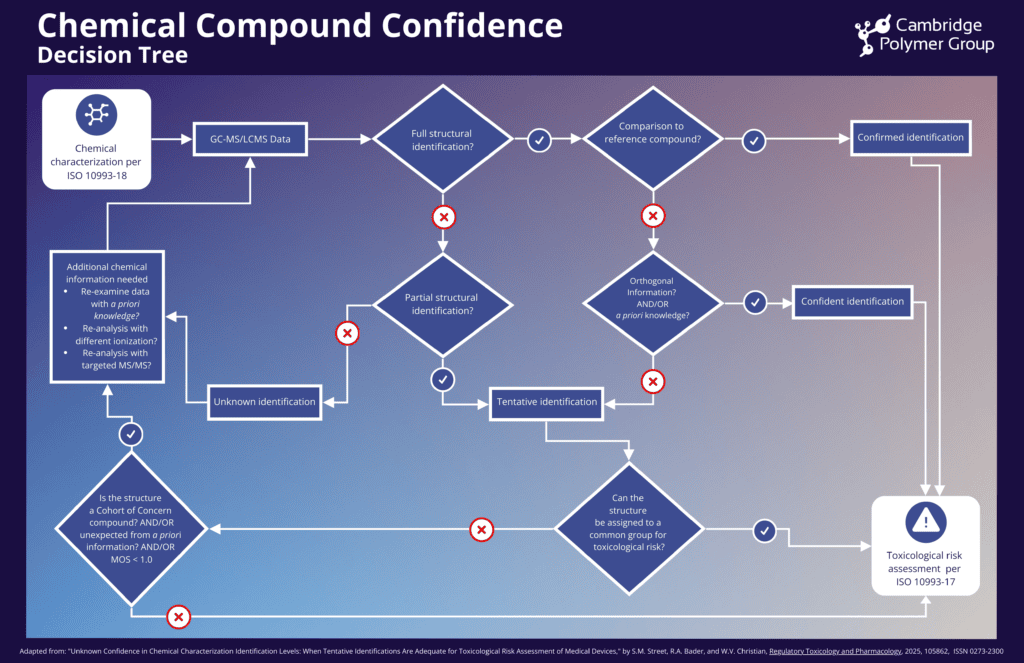
As highlighted in the recent article, “Unknown Confidence in Chemical Characterization Identification Levels: When Tentative Identifications Are Adequate for Toxicological Risk Assessment of Medical Devices,” are common: a review of approximately 600 chemical characterization reports from a range of laboratories, including manufacturer-operated analytical facilities and contract research organizations, found that about 43% of reported organic compounds were only tentatively identified. Although there has been a recent push for confirmed or confident identifications for toxicological risk assessment, ISO 10993-17 does not specify how to handle varying confidence levels. As such, through collaboration between toxicologists and chemists, a pragmatic approach that balances analytical rigor with practical constraints is possible.
Rather than evaluating every compound independently, the article highlights grouping chemicals with similar structures for toxicological risk assessment. This approach allows for efficient evaluation of potential hazards, even when full identification is not possible. To support this method, the authors developed a decision tree that encourages early communication between chemists and toxicologists and helps analysts determine when additional analytical information is needed to improve compound identification. This structured process ensures that the toxicological risk posed by all chemicals can be assessed appropriately, including those of higher risk that may require a more detailed investigation.
Download the PDF version.
Co-Author Spotlight: Rebecca Bader
The article’s insights are informed by the expertise of co-author Rebecca (Becky) Bader, PhD, Director of Regulatory Services at Cambridge Polymer Group. With over 20 years of experience in polymeric materials, drug delivery, and analytical chemistry, Becky brings deep expertise from both industry and academia. Her leadership at CPG is instrumental in advancing analytical techniques and ensuring that chemical characterization studies meet the highest standards of scientific rigor and regulatory compliance.
Join Our Upcoming Webinar The Tightrope of Tentative IDs: Balancing Analytical Uncertainty with Toxicological Risk Assessment
July 9, 2025 | 2:00 PM EDT
Don’t miss this opportunity to dive deeper into balancing analytical uncertainty and toxicological risk! Study authors Becky Bader and Steph Street, a Senior Principal Toxicologist and Biocompatibility SME for Medtronic, will host a webinar discussing:
- Regulatory expectations regarding compound identification and toxicological risk assessment
- The grouping of chemical compounds, including those with tentative identifications, for toxicological risk assessment
- Strategies for streaming lining the chemical characterization and toxicological risk assessment process
- Case studies on collaborative chemist-toxicologist workflows
Register now to secure your spot!
How Cambridge Polymer Group Can Help
Cambridge Polymer Group specializes in comprehensive material and chemical characterization services, including extractables and leachables testing for medical devices. Our team, led by experts like Becky, leverages analytical instrumentation and deep knowledge of polymer science to:
- Design and execute tailored extractables and leachables studies
- Identification of unknowns for toxicological risk assessment
- Provide clear, defensible reports for regulatory submission
- Guide clients through the decision-making process, from data collection to risk assessment and regulatory strategy
Every medical device is unique, and our approach ensures that analytical methods and risk assessments are customized to your product’s specific materials, manufacturing processes, and intended use. Whether you need targeted testing or a comprehensive chemical risk assessment, CPG’s experience and expertise can help you navigate the evolving regulatory landscape and bring safe, effective devices to market.