
ASTM Workshop on Hydrogel Characterization
ASTM International will host the Workshop on the Characterization of Hydrogel Medical Products on May 6, 2025, in Toronto, Canada, during the spring meeting of the ASTM Committee F04 on Medical and Surgical Materials and Devices. This event brings together leading experts to discuss best practices, emerging analytical techniques, and the urgent need for standardized testing methods […]
Microplastics in Infusion Bags
Microplastics have become a pressing topic in environmental and health discussions, with increasing attention from the media and scientific community. These tiny plastic particles, typically defined as ranging in size from 1 micrometer to 5 millimeters, can be composed of various types of polymers and are now being detected in an array of consumer products. A recent […]

Ensuring Trustworthy Third-Party Lab Data for Regulatory Success
Companies regularly rely on third-party laboratory testing data to support regulatory medical device and pharmaceutical submissions, particularly when lacking in-house expertise or facilities. The credibility of these third party laboratories is crucial to regulatory success, but recent actions by the FDA highlight the risks associated with unvetted or noncompliant third party data. Escalating FDA Scrutiny […]
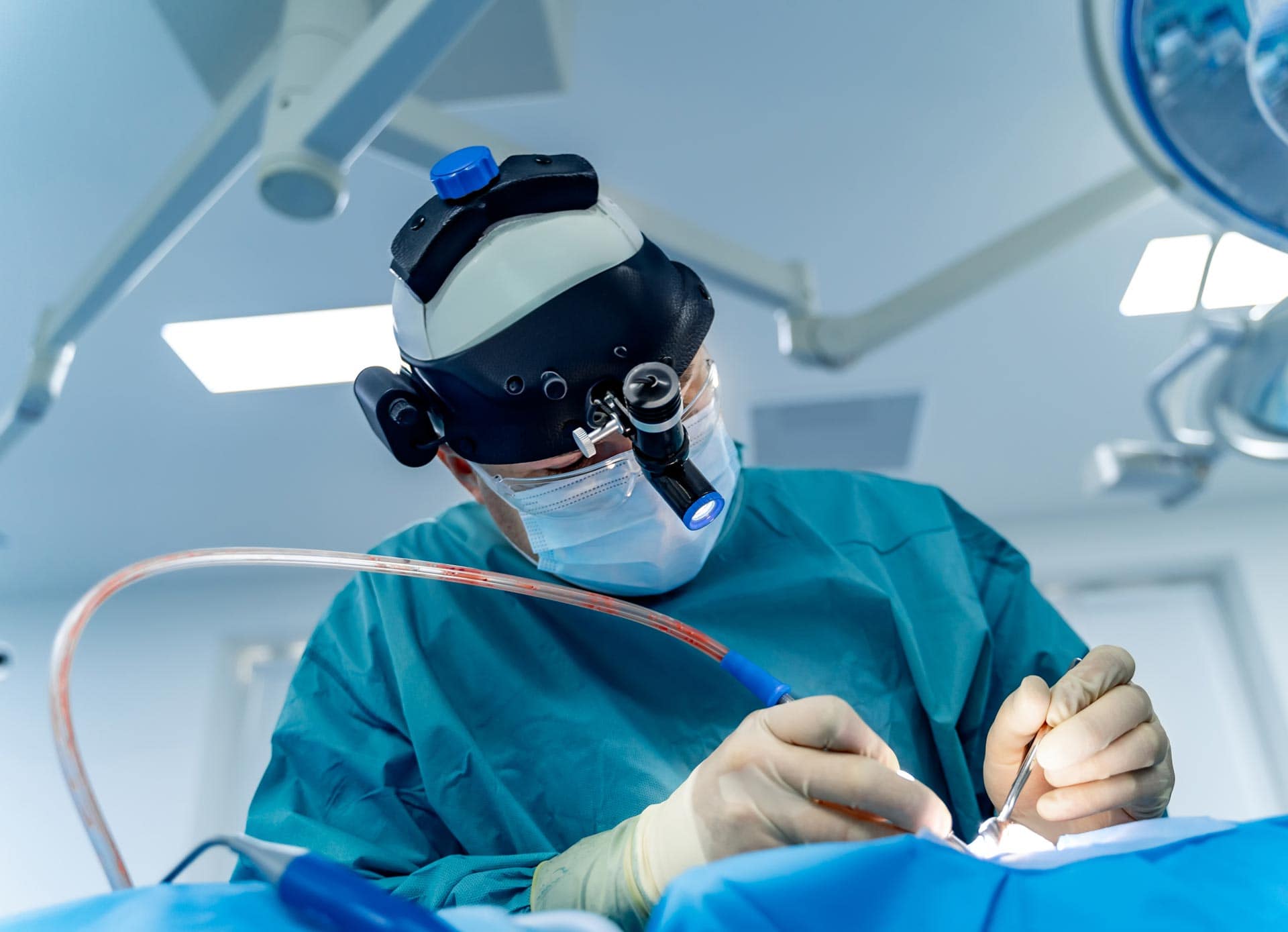
Thoughtful Design in Surgical Lighting: Balancing Usability, Durability, and Sustainability
Traditional headlamps (worn above) are cumbersome and don’t accommodate face shields. A groundbreaking surgical task light has been introduced by MezLight in collaboration with Syensqo[1], demonstrating a thoughtful approach to product design by considering key factors such as: Customer needs Sustainability concerns Environmental durability Material suitability Addressing Customer Needs: Enhanced Usability and Safety Traditional surgical […]
