Hip Implant Recall
Johnson & Johnson has continued to investigate their metal-on-metal implants, which were recalled in 2010 due to some patients reactions to metal debris generated during articulation. In a Reuter’s report today, J&J had fourth quarter charges of $800 million associated with medical costs related to the recall.

Radiopacity in Medical Devices
Temporary or permanent implants often contain a radiopacifier, which is a material with a higher electron density contrast compared to the surrounding material so that it absorbs X-ray energy. In an X-ray, a radiopacifier appears as a bright section, as shown in the catheter above (the internal wire is a radiopacifier). Radiopacifiers are often made […]
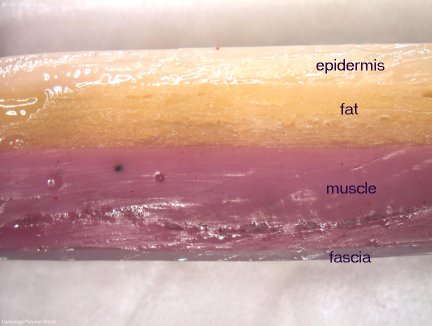
Hydrogel Skin Model
Synthetic tissue constructs have been around since the 1970’s, when Dr.’s Yannas and Burke created an artificial skin from collagen and silicone rubber. This membrane, termed Silastic, was designed to mimic the properties of skin, to help generate new skin in burn victims. Researchers from the Medical School Hannover (Germany) are trying to replicate human […]
Highly Crosslinked UHMWPE Available for License
Cambridge Polymer Group and Massachusetts General Hospital have co-developed novel, highly crosslinked ultra high molecular weight polyethylenes that incorporate vitamin E and are suitable for hip, knee, shoulder and spine arthroplasty applications. These technologies, generically termed CIMA, E-CIMA and Reservoir Vitamin E, are available for license. E-CIMA E-CIMA is a formulation containing Vitamin E throughout […]
Annual Meeting of the Orthopedic Research Society
CPG will have an exhibit at the upcoming annual meeting of the Orthopedic Research Society in San Francisco, CA, from February 4th to February 7th. This conference brings researchers, medical device manufacturers, surgeons, and regulatory agency representatives together to discuss the latest technologies, practices, and clinical outcomes in the area of orthopedic surgery, including hip, […]
