Potential Detection System for Implant Wear
Researchers at the University of Nebraska Medical center recently published their results of a study in which they developed an optical imaging agent to detect inflammation due to wear debris generated from implants. In this study, a N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-based optical imaging contrast agent (P-IRDye) was developed and used for the detection of wear particle-induced […]
Design for Cleaning
The FDA has issued a mandate to manufacturers of re-usable medical devices to design with re-cleaning of the these devices in mind. This mandate is in response to an on-going effort to reduce the risk of infection, contamination, or disease transmission due to improperly cleaned devices. Device design should include smooth interior surface, avoidance of […]
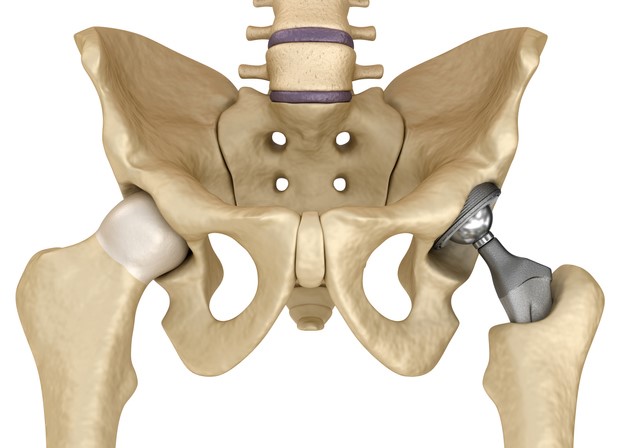
Metal-On-Metal Hip Implants
The on-going discussion of patient response to metal-on-metal hip implants has recently increased. For the past few years, papers presented at ORS and AAOS have discussed concerns about tissue response to metal ions and debris liberated from wear in metal-on-metal hip implants. These articles allege that the metal ions can result in pseudo-tumors in some […]

The FDA Will Be Hosting a Public Workshop on the Reprocessing of Reusable Medical Devices
The FDA will be hosting a public workshop on the Reprocessing of Reusable Medical Devices on June 8th and 9th, 2011 in Silver Springs, MD. The purpose of the workshop is to discuss factors affecting reprocessing quality, device design as it relates to reprocessing reusable medical devices, reprocessing methodologies, validation methodologies, healthcare facility best practices, […]
