Metal-on-Metal Hip Implants
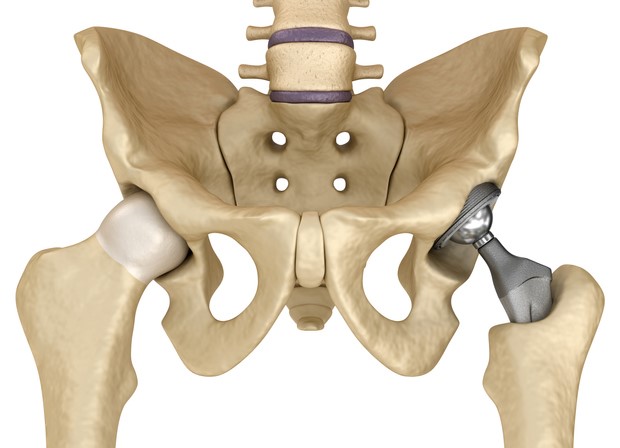
The on-going discussion of patient response to metal-on-metal hip implants has recently increased. For the past few years, papers presented at ORS and AAOS have discussed concerns about tissue response to metal ions and debris liberated from wear in metal-on-metal hip implants. These articles allege that the metal ions can result in pseudo-tumors in some patients. The FDA has recently requested that all orthopedic manufacturers who produce a metal-on-metal (MOM) implant conduct clinical studies on patients who have received a MOM implant. A NY Times article discusses the nature of this request. A recent NPR story also talks about issues with MOM, and Depuy's recent recall of their ASR system.