Organic solvents are commonly used as processing aids during the manufacture of polymer-based medical devices and related products. These volatile or semi-volatile solvents are not only used during product manufacturing but may also be used as cleaning agents for the products. Often complete removal of these residual solvents is not possible, and characterization of residual solvent levels in a product is a critical component of medical device risk assessment. To meet the detection requirements for ISO-10993 risk assessment, Cambridge Polymer Group (CPG) offers sensitive GC-MS analysis for residual solvents in medical devices. CPG is also well equipped to provide sensitive GC-MS analysis to meet USP and ICH guideline requirements for residual solvent levels in pharmaceutical products.
For unusual solvents or matrices, CPG can assist in the development, validation, and (where necessary) transfer of residual solvent analytical methods.
CPG scientists have experience developing and validating methods addressing a variety of residual solvent analytical challenges. Figure 1 shows an excerpt of a GC-MS method for the ppb-level trace analysis and quantitation of three xylene isomers and ethylbenzene. Figure 2 shows an example of an analytical method for the simultaneous separation and quantitation of 22 residual solvent target analytes.
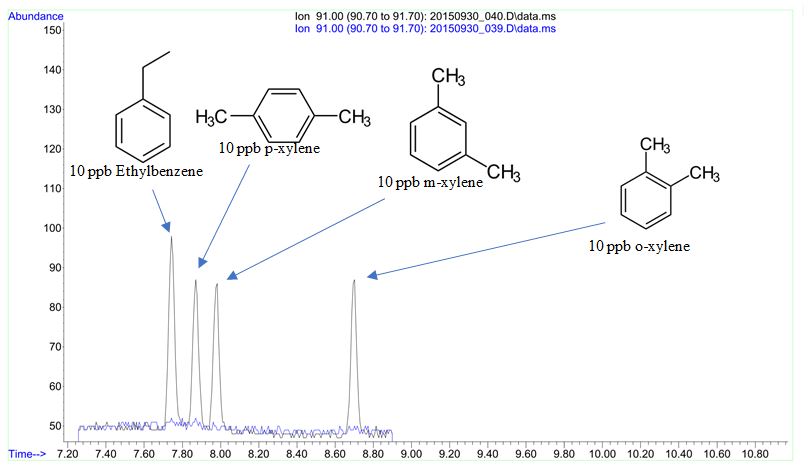
Figure 1: Selective ion chromatogram demonstrating separation, baseline resolution and trace (10ppb) detection of xylene isomers and ethylbenzene.
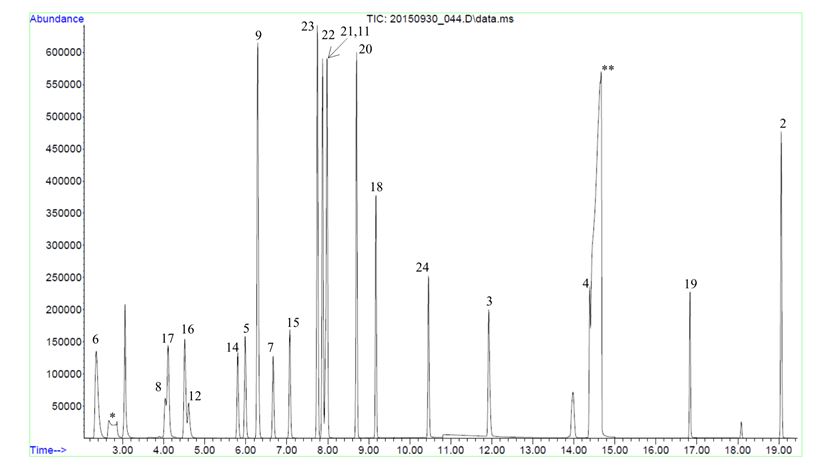
Figure 2: Total ion chromatogram demonstrating chromatographic method for the separation of 22 residual solvent target analytes. Some highly related species exhibit minor chromatographic co-elution but may be separated and quantified by characteristic mass spectral ions. Asterisks indicate matrix peaks.
Contact us to learn about how CPG’s residual solvent testing capabilities can assist in your current or future project work.
