
Caliente Chromatography: Quantitative Analysis of Capsaicin
Chromatography: To Quantify or Not to Quantify, That Is the Question Our chromatography team is regularly asked to identify compounds in materials. Some projects only require the identification of compounds, while others require the accurate determination of concentration of the identified compounds. The latter, termed quantitative chromatography, requires the preparation of calibration standards suitable for […]
 Abandoned plastic garden chair with broken leg, outdoor shot
Abandoned plastic garden chair with broken leg, outdoor shot
How Long Can a Polymer Last…And What Is Q10?
The longevity of polymers in real-world use is a critical importance across industries, from medical devices and packaging to consumer products and infrastructure. While many polymers are engineered to withstand sunlight, heat, moisture, and chemical exposure, nearly everyone has seen a once-flexible product turn brittle, discolored, or cracked over time. Both raw materials (such as […]
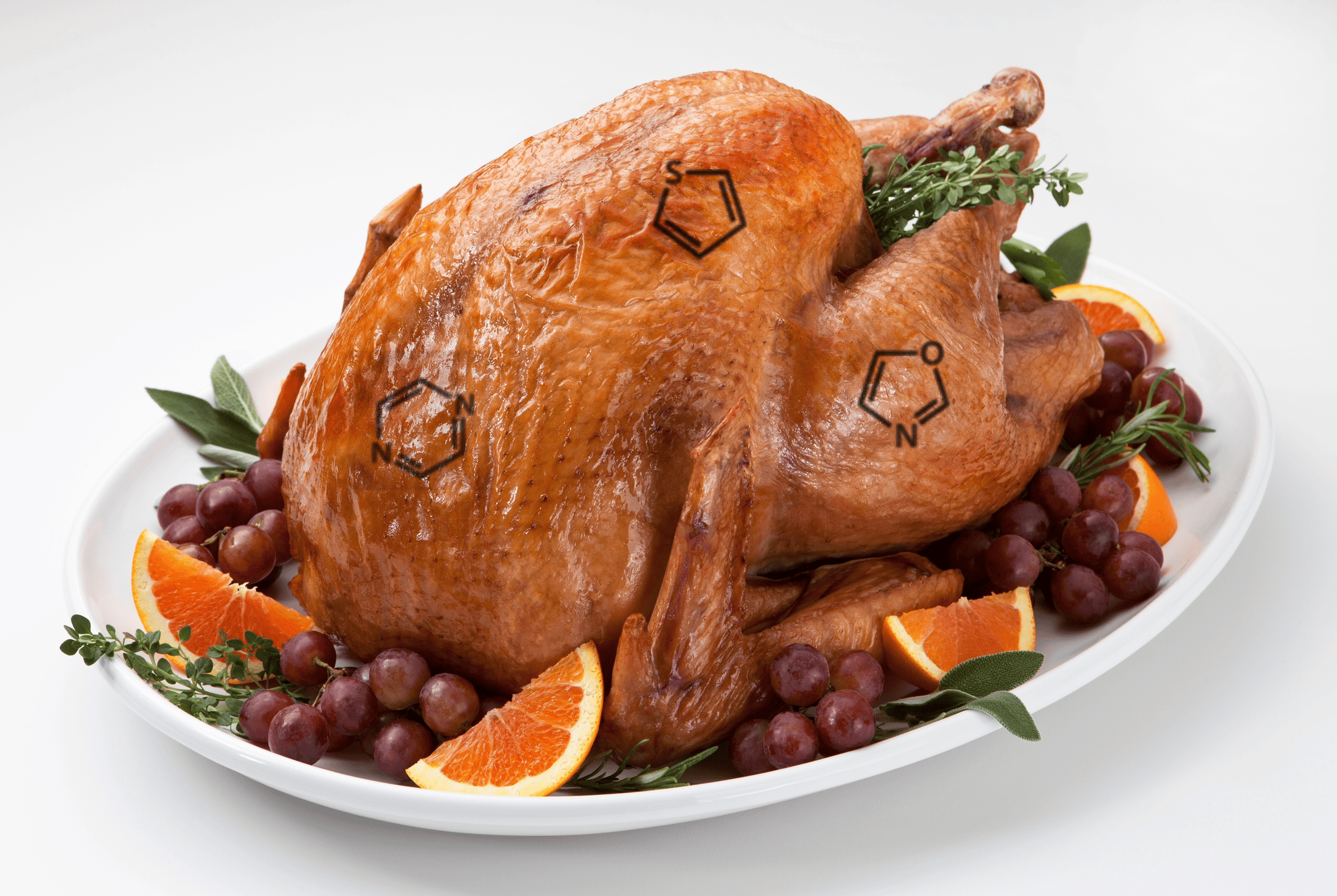
The Chemistry Behind the Perfect Roast: Understanding the Maillard Reaction
Every time you roast a turkey or bake bread, a fascinating chemical reaction gives your food its rich brown color, enticing aroma, and complex flavors. That reaction is called the Maillard reaction (pronounced my-ard), a cornerstone of both food chemistry and polymer science. What Is the Maillard Reaction? The Maillard reaction occurs when amino acids […]

From Residues to Risk: Why Medical Device Cleaning Validation Matters in Biocompatibility Assessments
Webinar – October 15, 2025, 2 p.m. ET Register Here Ensuring medical device safety requires a coordinated approach across three critical domains: biocompatibility, cleanliness, and sterility. While each area has its own regulatory and testing requirements, they are deeply interconnected. Overlooking the relationship between these elements can increase patient risk and delay product approvals. This upcoming medical […]

Hydrogel Water Beads: For Farming Use Only
What Are Water Beads? “Water beads” are water-absorbent polymer beads that can swell over several hundreds of times their initial dry mass when placed in water, forming hydrogel beads. They are primarily marketed as agricultural products to act as humectants in soil, helping retain and slowly release water into the soil. Beyond farming, however, water […]
Flamingos Doing Vector Calculus
While we’ve previously celebrated the kitschy charm of plastic flamingos, today we turn our attention to the remarkable living birds and the science behind their mesmerizing feeding behaviors. With their beaks and most of their heads submerged near their feet, the birds stomp their feet in a rhythmic manner while chattering their beaks. But what […]

Potential Changes to the Generally Recognized as Safe (GRAS) Program
Background: The GRAS Framework The Food Additives Amendment to the Federal Food, Drug, and Cosmetic Act (FD&C Act) was established by Congress in 1958. In the Code of Federal Regulations, the rules that the FDA applies to food additives are spelled out in sections 21 CFR 170.3 and 170.30. A food additive is considered to […]
