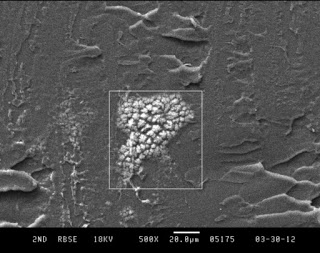
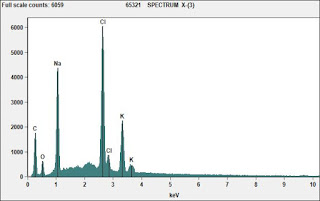
Tensile strength of fluids

Authors Lubansky et al from Swansea University in the UK analyzed the tensile strength of polyethylene glycol fluids using a CaBER(r) capillary breakup extensional rheometer. In this technique, a small aliquot of fluid is stretched rapidly between two endplates, and the resultant filament breakup kinetics are monitored with a high resolution laser micrometer. The breakup kinetics are a function of the fluids extensional rheological properties, coupled with surface tension. These properties can influence the behavior of fluids in jetting flows, fiber spinning, liquid deposition, and a variety of other processing methodologies.
The article was published in the Journal of Non-Newtonian Fluid Mechanics, and can be accessed here.
More information on the CaBER(r) can be found here.