Swedish warships and quality management systems

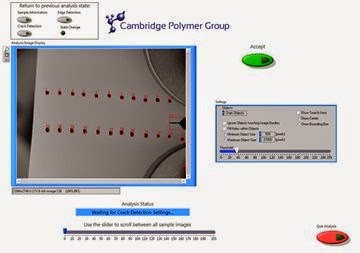
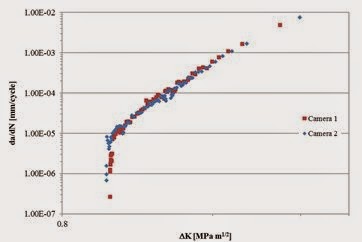
In the design of a new device, a good manufacturer will follow quality management principles to ensure the device meets the requirements of the end-user. What does this have to do with Swedish warships? History can provide us with useful lessons on quality management systems. The Vasa was a warship built in 1626 that holds the unfortunate reputation of sinking on her maiden voyage after sailing less than a mile. From a modern perspective, the construction and launch of the Vasa suffered from one primary problem. The lack of a quality management system during her design meant that a good design team was not assembled and new design concepts were not adequately tested and verified during construction. As a result, design changes were instituted “on the fly” after the nominal design freeze, and contradicting results from final verification tests were ignored, or never reached the right people. As a consequence, she was unable to meet the specifications required to be sea-worthy, and sank quickly when a gust of wind hit her when leaving Stockholm's harbor an incorrect weight balance resulting from overly complex superstructure. An adequate quality management system might have provided the checks and balances and gating necessary to any good design process, perhaps avoiding her sinking.
As an aside of a more polymeric nature, she was raised piece by piece from the sea floor in 1961, and currently sits in a museum in Stockholm, where she is in surprisingly good shape. She is being impregnated with polyethylene glycol as a preservative, although recent studies are suggesting that PEG application to wood in an acidic environment (the Vasa was in acidic water for centuries) will form formic acid, which could damage the wood.
Download full application note